Inflammation in severe asthma
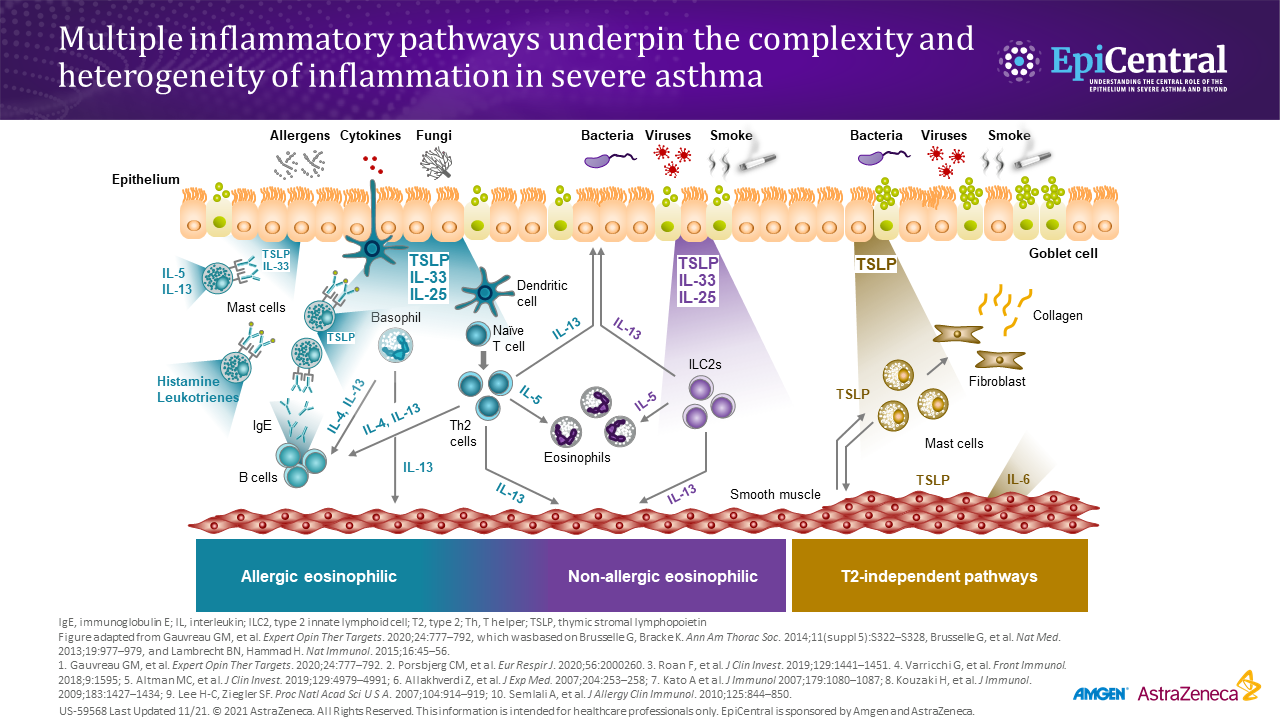
Inflammation in asthma leads to increased symptoms.1,5 Asthma-associated inflammation is complex and heterogeneous,1–4 and numerous cell types, mediators, and downstream immune pathways are involved.1–6 Multiple inflammatory endotypes have been characterized – allergic and eosinophilic inflammation, to name a few.6