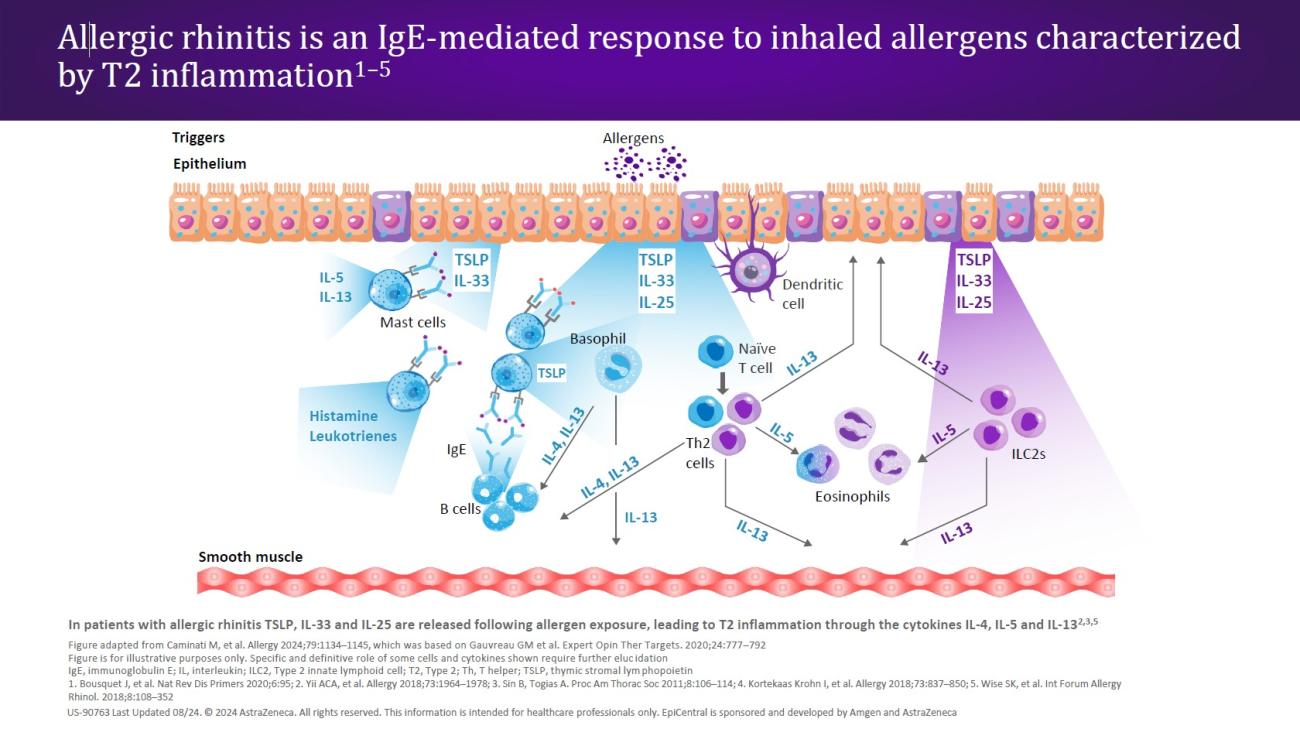
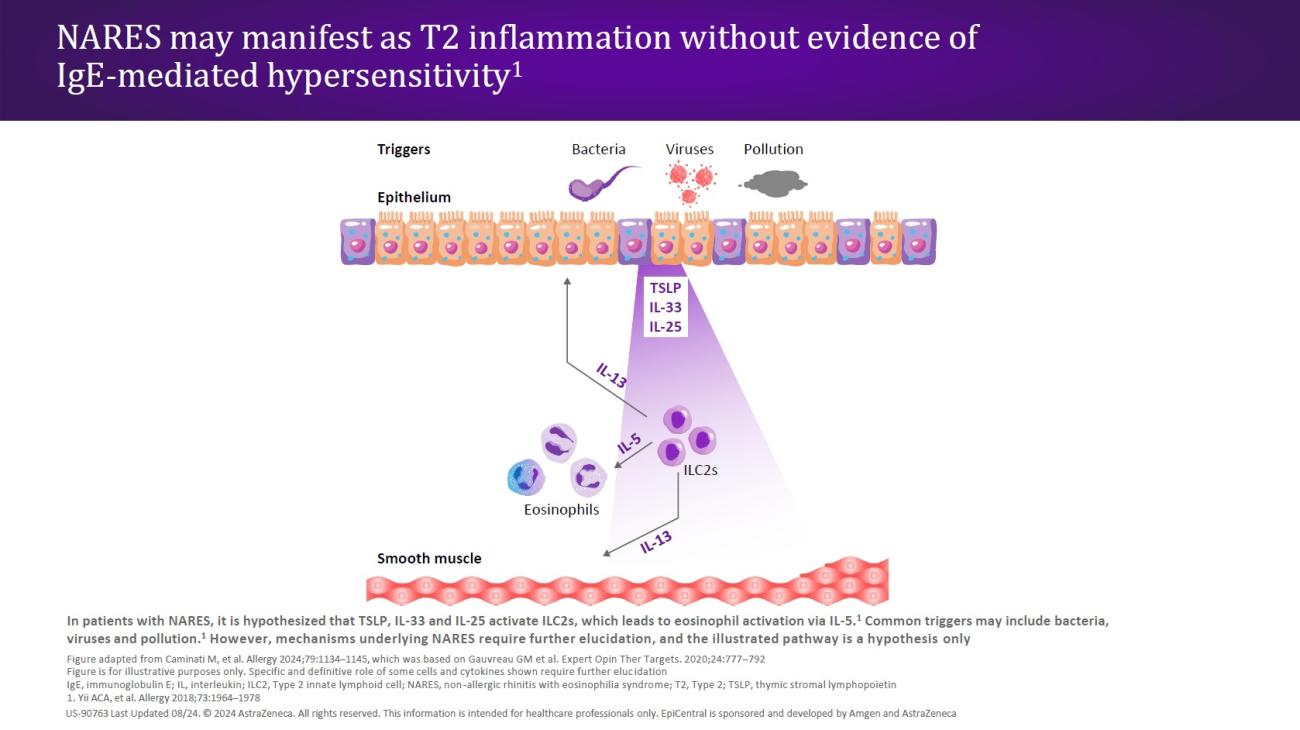
CRSwNP
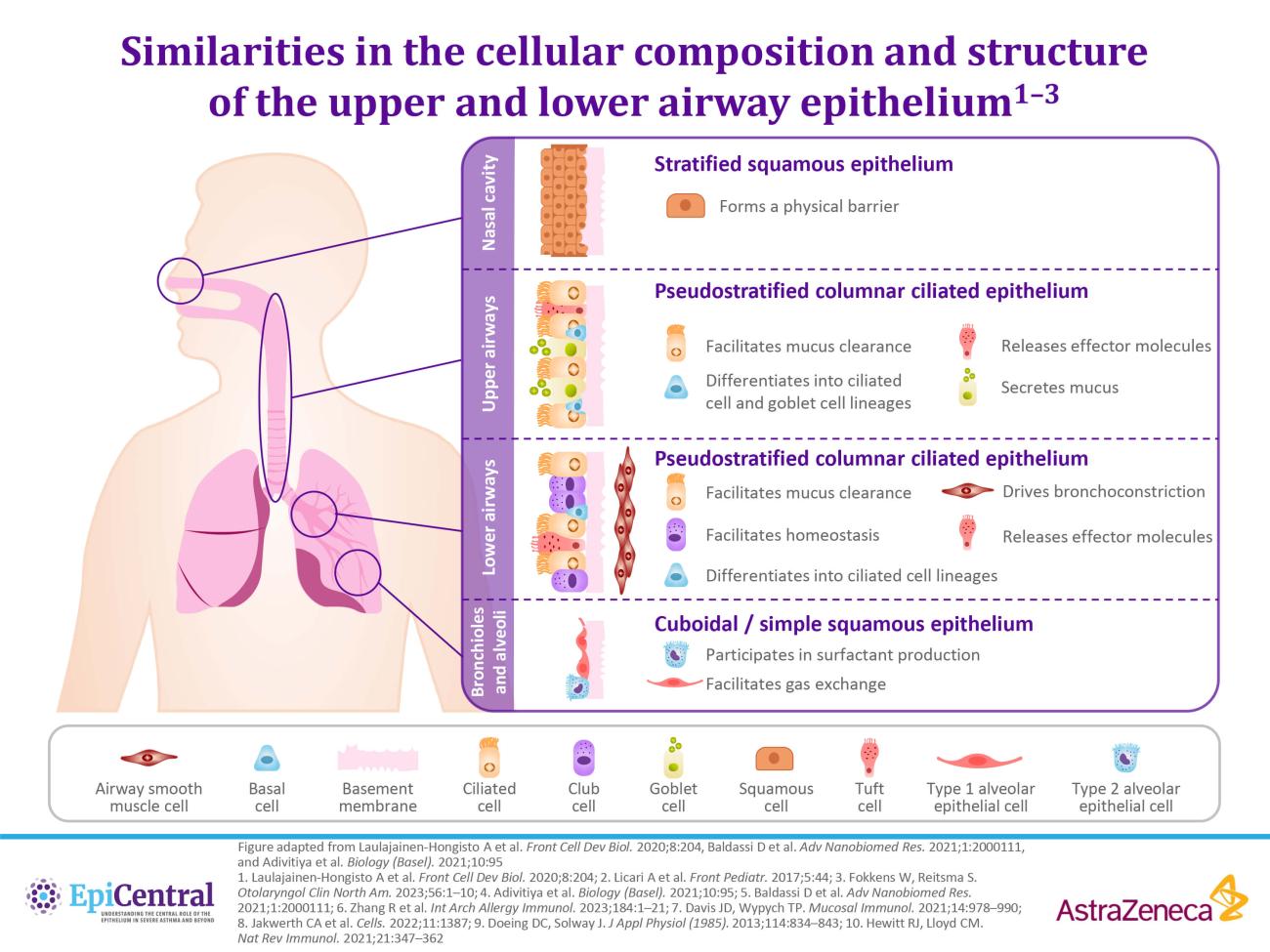
Nasal polyps are gray masses within the nose or sinuses that originate from the ethmoid sinus and project into the nasal cavity.16,26 CRSwNP is characterized by nasal congestion, a decreased sense of smell and taste, and sleep disruption.9,13,27 These symptoms place a substantial burden on patients, severely impacting their quality of life.9,27,28 Patients report negative impacts on their psychological and social wellbeing due to decreased enjoyment of food, embarrassment about their symptoms (ie, nasal discharge), and poor sleep quality.9,28
Key unmet needs remain for patients with CRSwNP, including feeling that the burden of disease they experience is underestimated by healthcare providers, and the ineffectiveness of current available treatments.28
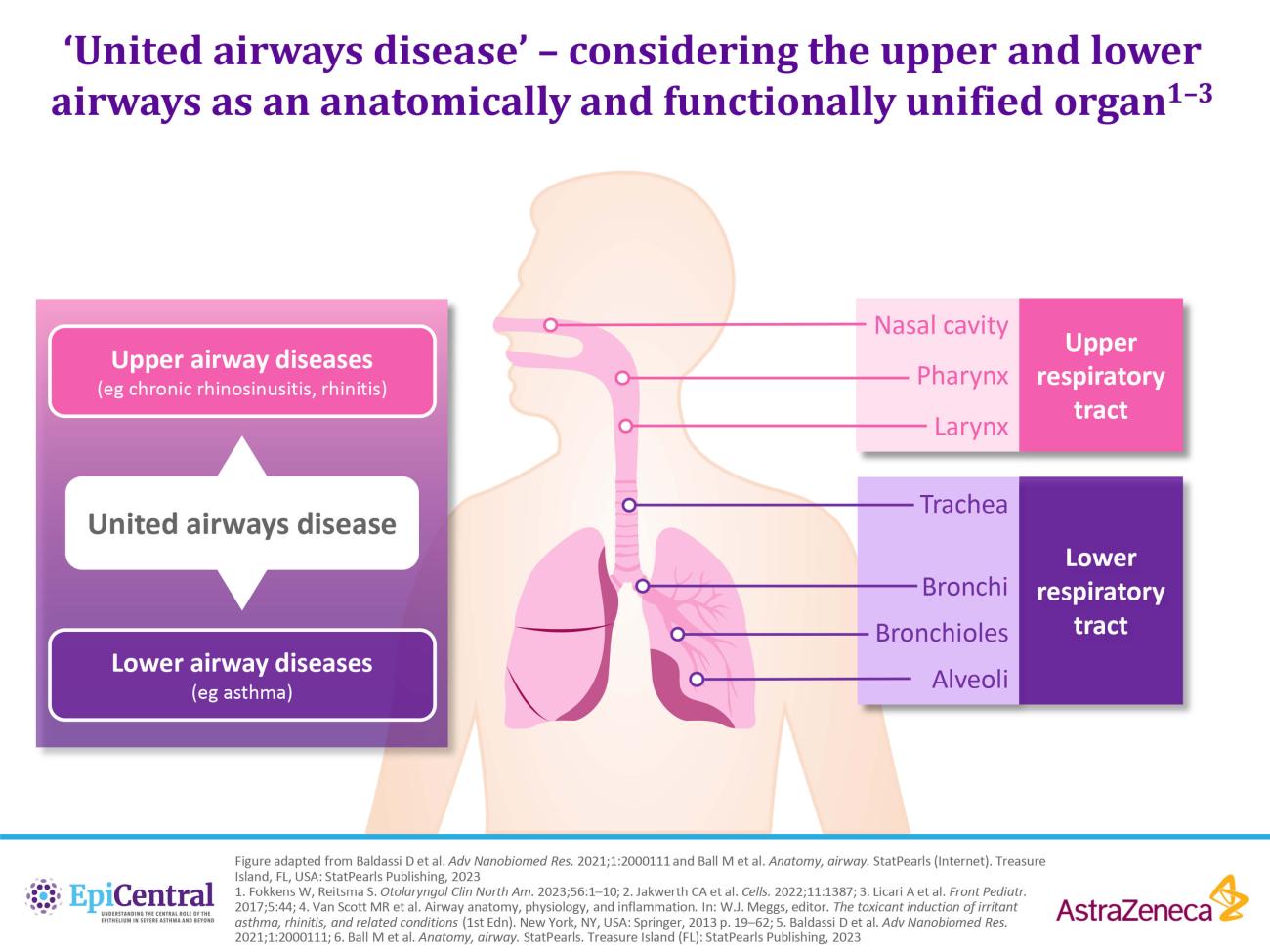
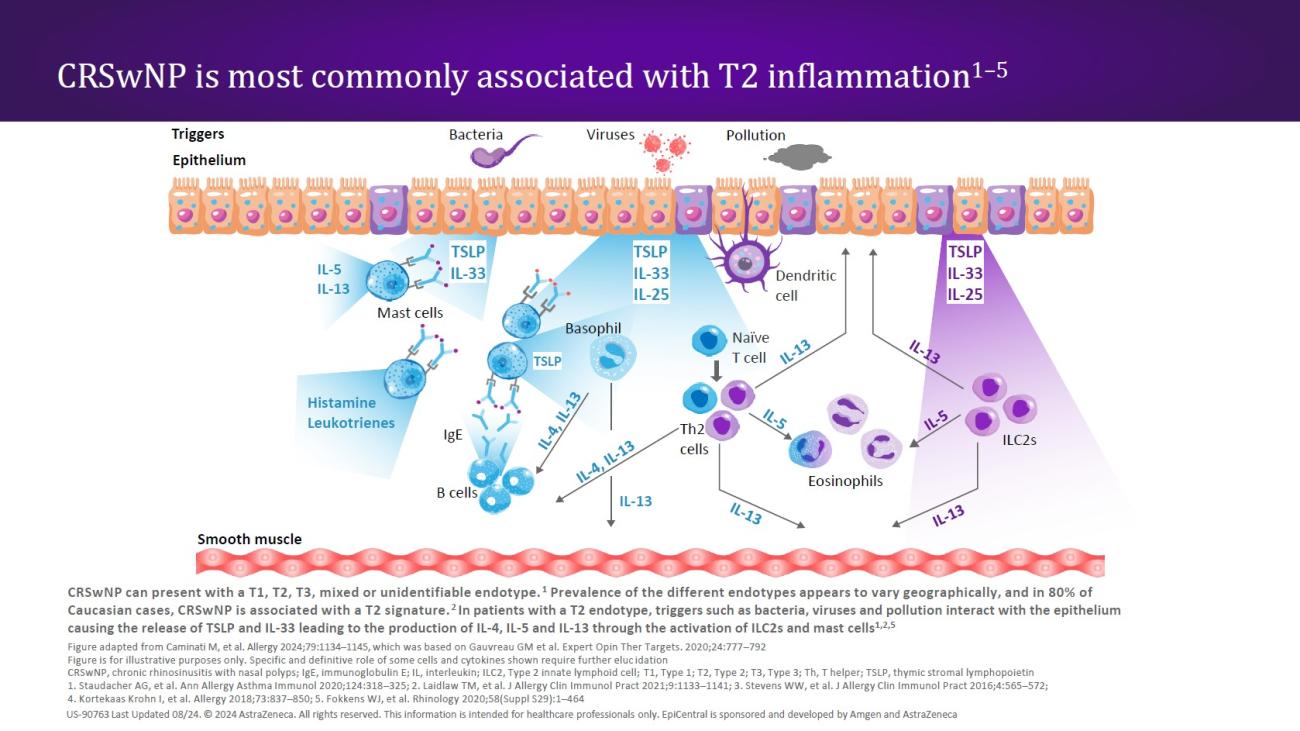
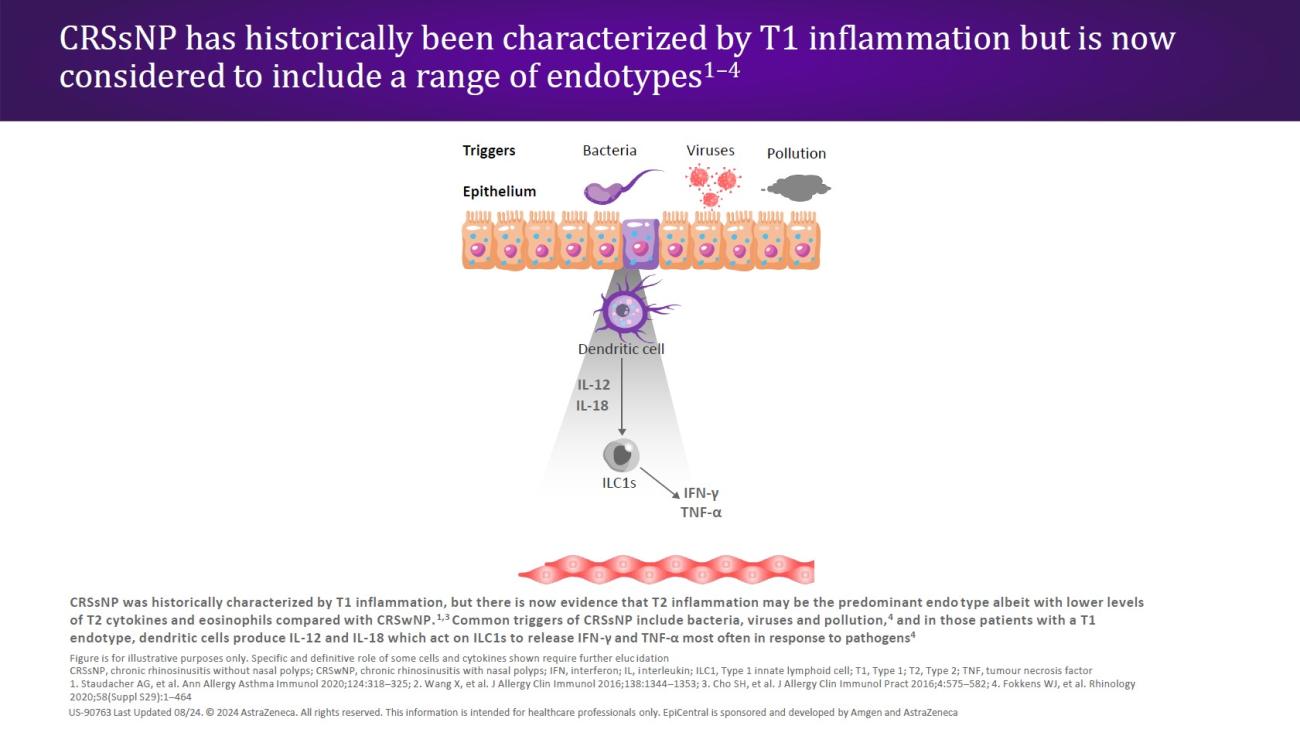
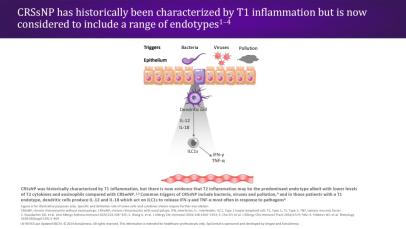
CRSwNP is most commonly associated with a Type 2 (T2) endotype, although many patients show evidence of a mixed endotype.25,29 Endotypes vary geographically24; while T2 inflammation is the predominant endotype in Western countries,25,29 Asian countries may have higher proportions of Type 1 (T1) and Type 3 (T3) disease.24,29 In recent decades, there has been an increase in the proportion of T2 endotypes in Asian countries, which may be caused by increasingly Western lifestyles in this region.24
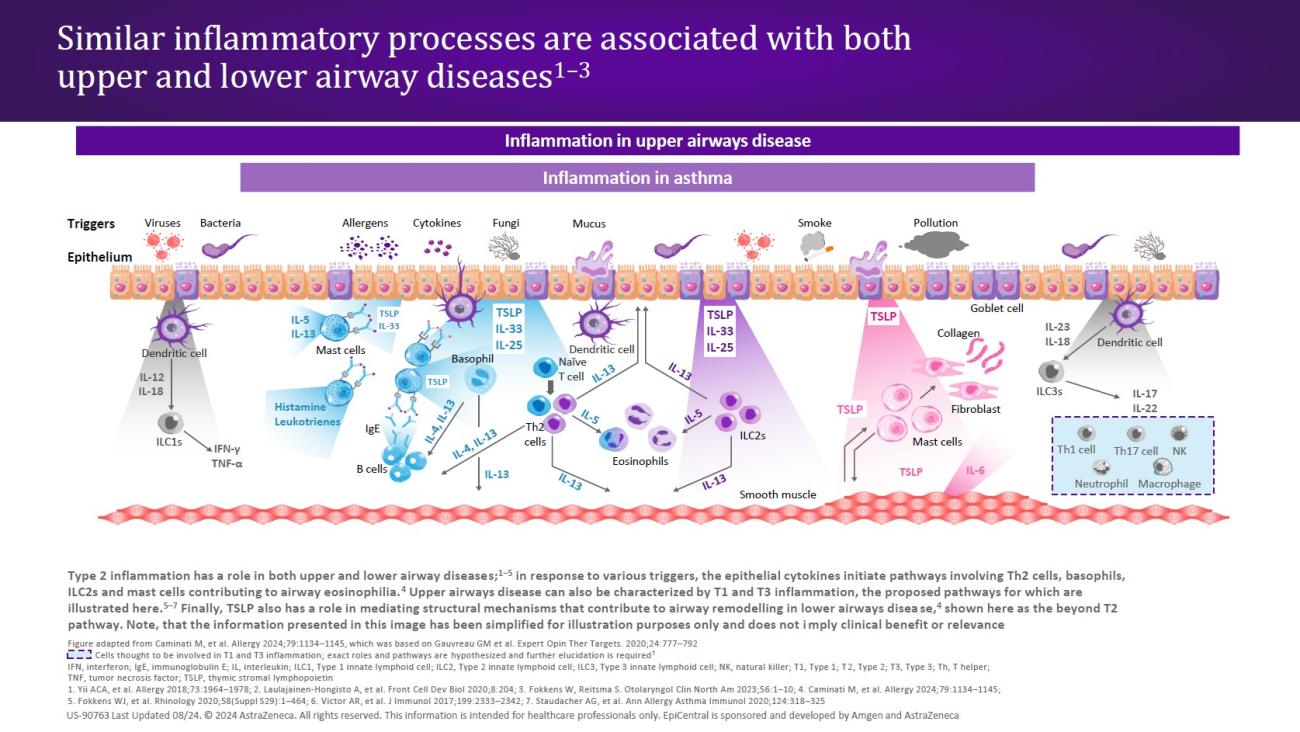
In patients with a T2 endotype, epithelial disruption leads to a cycle of T2 inflammation.30 The defective epithelial barrier is more easily permeable, and epithelial cytokines (TSLP, IL-33, and IL-25) are released in response to external triggers acting on the epithelium.16,30 All three epithelial cytokines have been shown to be overexpressed in patients with CRSwNP.31–33 The release of TSLP and IL-33 in turn leads to the production of downstream mediators (ie, IL-4, IL-5, and IL-13) through the activation of innate Type 2 lymphoid cells (ILC2s) and mast cells24,30,33; ILC2s, mast cells, and the T2 cytokines IL-5 and IL-13 have all shown to be elevated in patients with CRSwNP.16 These cytokines amplify the T2 inflammatory response,30,33 while IL-4 and IL-13 may perpetuate barrier dysfunction through induction of additional TSLP expression.30
In patients with a T2 endotype, the release of TSLP and IL-33 leads to the production of IL-4, IL-5, and IL-13 through the activation of ILC2s and mast cells.24,30,33