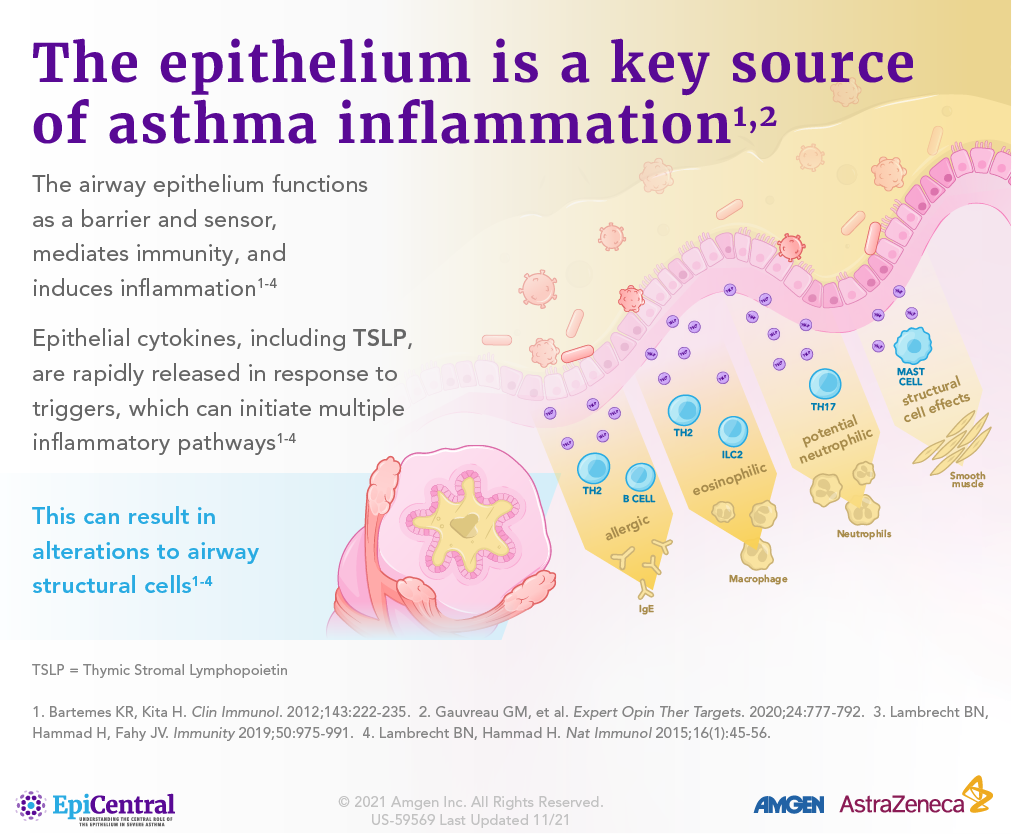
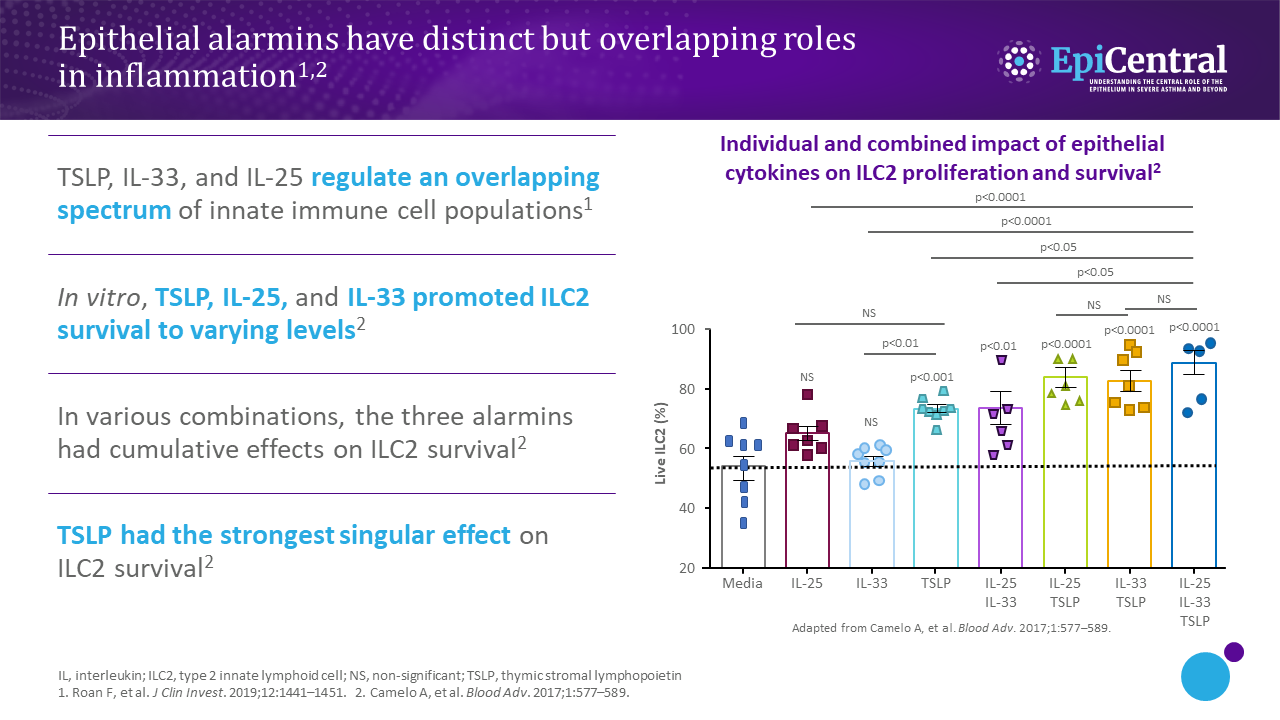
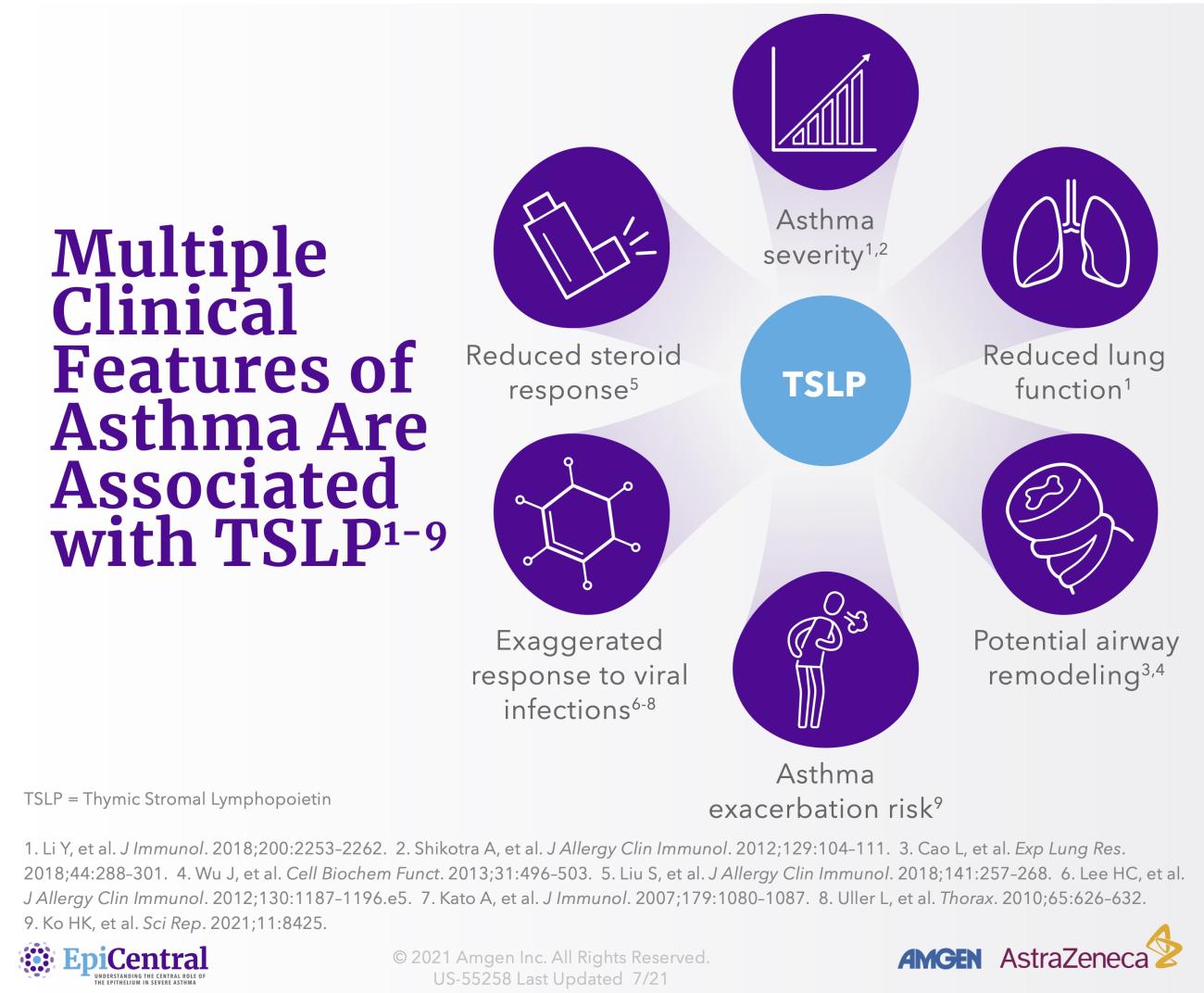
TSLP, IL-33, and, to a lesser extent, IL-25 have a large number of cellular targets.1 IL-33 targets myeloid dendritic cells, CD4+ T cells, CD8+ T cells, regulatory T cells, natural killer T cells, mast cells, macrophages, B cells, eosinophils, basophils, neutrophils, type 2 innate lymphoid cells (ILC2s), airway epithelium, and fibroblasts.1 TSLP targets myeloid dendritic cells, CD4+ T cells, CD8+ T cells, regulatory T cells, natural killer T cells, B cells, mast cells, monocytes, eosinophils, basophils, ILC2s, and the airway epithelium.1 IL-25 targets ILC2s, CD4+ T cells, invariant natural killer T cells, airway epithelial cells, and fibroblasts.1