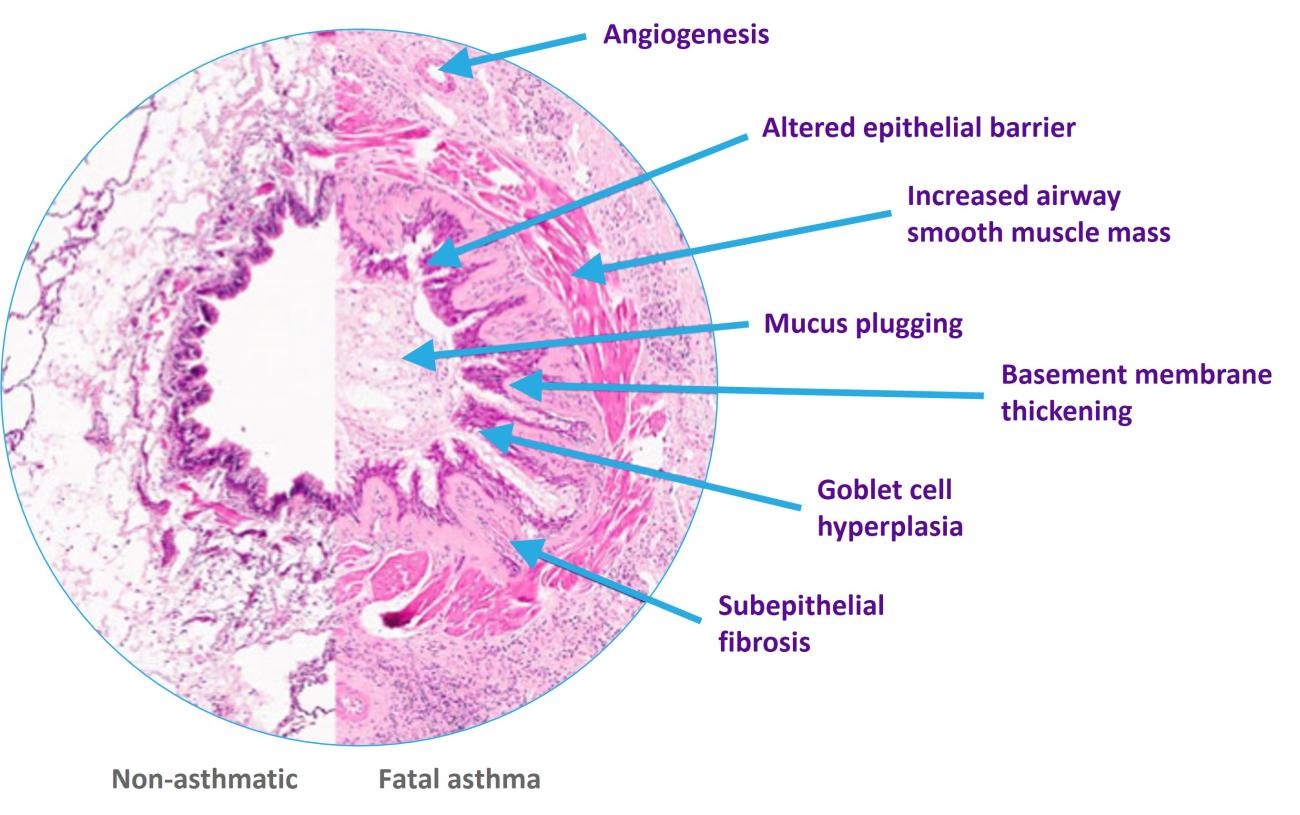
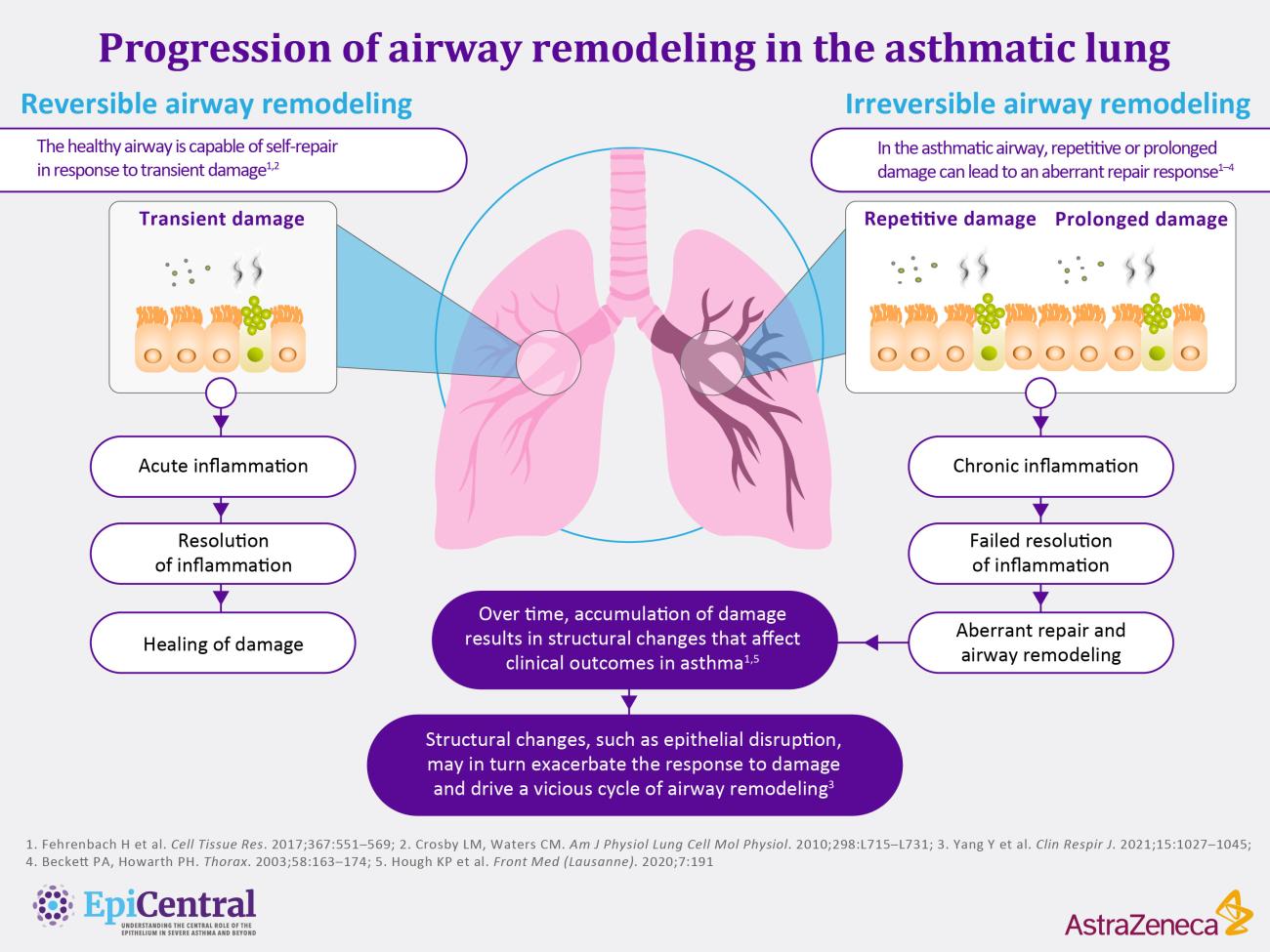
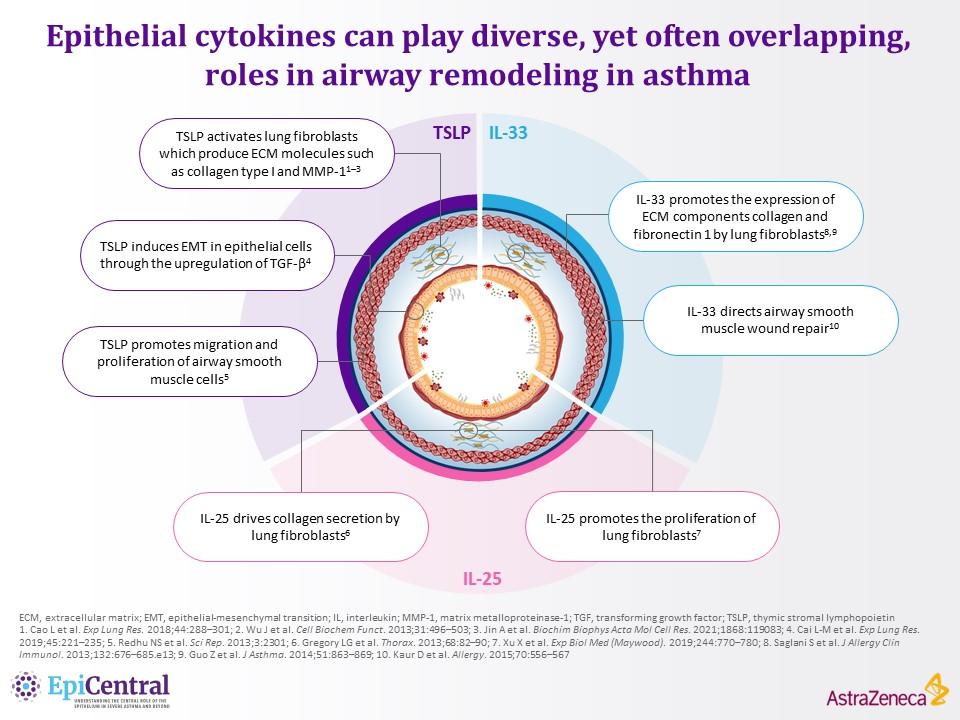
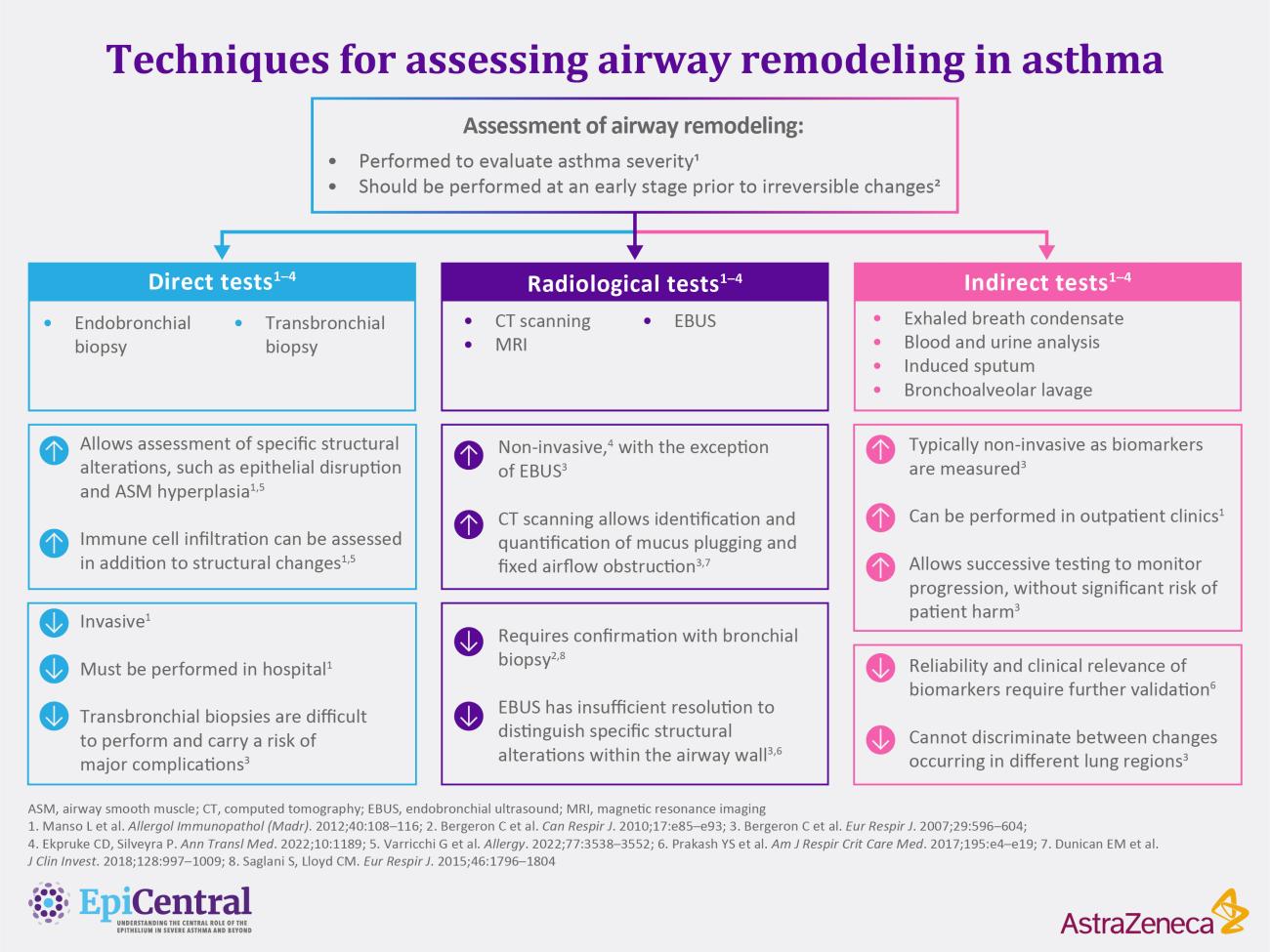
References
1. Hough KP, et al. Front Med (Lausanne). 2020;7:191. 2. Yang Y, et al. Clin Respir J. 2021;15:1027–1045. 3. Beckett PA, Howarth PH. Thorax. 2003;58:163–174. 4. James AL, Wenzel S. Eur Respir J. 2007;30:134–155. 5. Hsieh A, et al. Front Physiol. 2023;14:1113100. 6. Varricchi G, et al. Allergy. 2022;77:3538–3552. 7. Samitas K, et al. Allergy. 2018;73:993–1002. 8. Gauvreau GM, et al. Allergy. 2023;78:402–417. 9. Thomas D, et al. Eur Respir J. 2022;60:2102583. 10. Fehrenbach H, et al. Cell Tissue Res. 2017;367:551–569. 11. Brightling CE, et al. Clin Exp Allergy. 2012;42:638–649. 12. Krings JG, et al. J Allergy Clin Immunol. 2021;148:752–762. 13. Zhang J, Dong L. J Thorac Dis. 2020;12:6090–6101. 14. Gras D, et al. Med Sci (Paris). 2011;27:959–965. 15. Gupta S, et al. Chest. 2009;136:1521–1528. 16. Calvén J, et al. Int J Mol Sci. 2020;21:8907. 17. Heijink IH, et al. Allergy. 2020;75:1902–1917. 18. Davies DE. Proc Am Thorac Soc. 2009;6:678–682. 19. Witt CA, et al. Acad Radiol. 2014;21:986–993. 20. Barbato A, et al. Am J Respir Crit Care Med. 2006;174:975–981. 21. Saglani S, et al. Am J Respir Crit Care Med. 2007;176:858–864. 22. Saglani S, Lloyd CM. Eur Respir J. 2015;46:1796–1804. 23. Takizawa H. Respir Med CME. 2008;1:69–74. 24. Joseph C, Tatler AL. J Asthma Allergy. 2022;15:595–610. 25. Dunican EM, et al. Ann Am Thorac Soc. 2018;15:S184–S191. 26. Chanez P, et al. Am J Respir Crit Care Med. 1999;159:588–595. 27. Fokkens W, Reitsma S. Otolaryngol Clin North Am. 2023;56:1–10. 28. Sozener ZC, et al. Allergy. 2022;77:1418–1449. 29. Bartemes KR, Kita H. Clin Immunol. 2012;143:222–235. 30. Jackson DJ, Lemanske RF Jr. Immunol Allergy Clin North Am. 2010;30:513–522, vi. 31. Crosby LM, Waters CM. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. 32. Grainge CL, et al. N Engl J Med. 2011;364:2006–2015. 33. Tschumperlin DJ, Drazen JM. Am J Respir Crit Care Med. 2001;164:S90–S94. 34. Chapman DG, Irvin CG. Clin Exp Allergy. 2015;45:706–719. 35. Comberiati P, et al. Immunol Allergy Clin North Am. 2018;38:545–571. 36. Yang D, et al. Immunol Rev. 2017;280:41–56. 37. Cao L, et al. Exp Lung Res. 2018;44:288–301. 38. Wu J, et al. Cell Biochem Funct. 2013;31:496–503. 39. Jin A, et al. Biochim Biophys Acta Mol Cell Res. 2021;1868:119083. 40. Saglani S, et al. J Allergy Clin Immunol. 2013;132:676-685.e13. 41. Guo Z, et al. J Asthma. 2014;51:863–869. 42. Gregory LG, et al. Thorax. 2013;68:82–90. 43. Xu X, et al. Exp Biol Med (Maywood). 2019;244:770–780. 44. Redhu NS, et al. Sci Rep. 2013;3:2301. 45. Kaur D, et al. Allergy. 2015;70:556–567. 46. Cai L-M, et al. Exp Lung Res. 2019;45:221–235. 47. Ojiaku CA, et al. Am J Respir Cell Mol Biol. 2017;56:432–442. 48. Osei ET, et al. Cells. 2020;9:1694. 49. Türkeli A, et al. Exp Ther Med. 2021;22:689. 50. Roan F, et al. J Clin Invest. 2019;129:1441–1451. 51. Porsbjerg CM, et al. Eur Respir J. 2020;56:2000260. 52. Galli SJ, Tsai M. Nat Med. 2012;18:693–704. 53. Robinson DS. J Allergy Clin Immunol. 2004;114:58–65. 54. Brightling CE, et al. N Engl J Med. 2002;346:1699–1705. 55. Suto W, et al. Int J Mol Sci. 2018;19:3036. 56. Woodman L, et al. J Immunol. 2008;181:5001–5007. 57. Comeau MR, Ziegler SF. Mucosal Immunol. 2010;3:138–147. 58. Saunders R, et al. Clin Transl Immunology. 2020;9:e1205. 59. Saunders R, et al. J Allergy Clin Immunol. 2009;123:376–384. 60. Tatler AL, et al. J Immunol. 2011;187:6094–6107. 61. Sutcliffe A, et al. Thorax. 2006;61:657–662. 62. Moir LM, et al. J Allergy Clin Immunol. 2008;121:1034–1039.e4. 63. Gras D, et al. Eur Respir J. 2017;49:1602399. 64. Bergeron C, et al. Can Respir J. 2010;17:e85–e93. 65. Manso L, et al. Allergol Immunopathol (Madr). 2012;40:108–116. 66. Prakash YS, et al. Am J Respir Crit Care Med. 2017;195:e4–e19. 67. Gautam Y, et al. J Pers Med. 2022;12:66. 68. Baldassi D, et al. Adv Nanobiomed Res. 2021;1:2000111.