Chronic rhinosinusitis (CRS) is a disease characterized by persistent sinonasal inflammation.2 It is divided into two main phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).2 There are other phenotypes of CRS, including aspirin-exacerbated respiratory disease (AERD), infectious rhinosinusitis, and fungal rhinosinusitis, a subtype of which is allergic fungal rhinosinusitis (AFRS).8
Chronic rhinosinusitis and the central role of the nasal epithelium
Epithelial dysregulation plays a central role in the pathogenesis of
chronic rhinosinusitis1
Epithelial dysregulation plays a central role in the pathogenesis of chronic rhinosinusitis1
Module synopsis
- Chronic rhinosinusitis (CRS) is characterized by chronic sinonasal inflammation and divided into two main phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP)2
- Estimates of CRS prevalence range from less than 5% of the population to more than 10%3
- Among patients with CRS, one study showed that approximately 20% have CRSwNP4
- The condition has a significant psychological and social burden, but patients perceive an underestimation of disease burden by healthcare professionals (HCPs)5
- Epithelial dysfunction is an important characteristic of CRS and impedes the ability of the epithelium to act as a physical and immune barrier against the external environment1
- Elevated expression of epithelial cytokines has been demonstrated in the nasal epithelium of patients with CRSwNP,6 and mRNA levels of thymic stromal lymphopoietin (TSLP), the TSLP receptor (TSLPR), and the interleukin (IL)-33 receptor (ST2L) have been shown to correlate with markers of disease severity in eosinophilic CRSwNP7
Professor Claire Hopkins' and Dr Anju Peters' thoughts on chronic rhinosinusitis and the central role of the nasal epithelium
Insights from our EpiCollaborators
Chronic rhinosinusitis (CRS) is a disease characterized by persistent sinonasal inflammation.2 It is divided into two main phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).2 There are other phenotypes of CRS, including aspirin-exacerbated respiratory disease (AERD), infectious rhinosinusitis, and fungal rhinosinusitis, a subtype of which is allergic fungal rhinosinusitis (AFRS).8
CRS has a substantial negative impact on health-related quality of life and represents a significant psychological and social burden.5,10 Patients report that symptoms, notably olfactory dysfunction and nasal congestion, have a major impact on daily activities, including reducing food enjoyment, reducing sleep quality, and causing feelings of embarrassment.5
In fact, the symptoms of CRS are perceived to result in a comparable health state to those of Parkinson’s disease or congestive heart failure, as defined by mean ShortForm SixDimension (SF-6D) impairment scores.10 Furthermore, compared with non-AERD CRSwNP or CRSsNP, data suggest that patients with AERD suffer the most burdensome symptoms.11
Additionally, several studies have shown an association between CRS and increased rates of depression and anxiety.12,13
Despite the significant impact of CRS, patients perceive an underestimation of disease burden by healthcare professionals (HCPs).5 Timely diagnosis and management are critical for patient outcomes and effective healthcare utilization; delayed surgical intervention for CRS is associated with greater postoperative healthcare needs.14,15 Yet, patients’ feelings of being dismissed can act as a barrier to effective communication with HCPs, which contributes to delays in diagnosis and treatment.16
In addition to clinical burden, CRS is associated with substantial healthcare resource utilization and economic impact.17 In a retrospective study (data obtained between 2013–2014), patients with CRSwNP and without CRSwNP were compared; the cohorts were matched by age, gender, asthma, chronic obstructive pulmonary disease (COPD) diagnosis, and Charlson Comorbidity Index. It was found that patients with CRSwNP incurred a mean annual total healthcare cost of $18964, which was 2.5 times higher than that incurred by those without CRSwNP.17
Current treatment algorithms for CRS recommend intranasal corticosteroids and/or saline rinses as the first-line treatment and as maintenance therapy.18 Subsequent treatment is advanced stepwise, through short courses of oral corticosteroids (OCS), endoscopic sinus surgery (ESS), and then revision surgery or, more recently, approved biologics.9,18
However, there are limitations to these approaches: OCS often provide only temporary alleviation of symptoms such as anosmia,19 and recurrent use of systemic corticosteroids (SCS) is associated with an increased risk of several adverse events, including pneumonia, osteoporosis, cardiovascular disease, cataracts, sleep apnea, depression/anxiety, and diabetes.20
Even appropriate medical therapy and surgery often do not achieve disease control in the long term:21 one study suggested that at least 40% of patients with CRS may have uncontrolled disease 3–5 years after functional ESS,22 and one study found that 35% of patients with CRSwNP who underwent ESS were found to have nasal polyp recurrence at 6-month follow up.23
Advances in understanding have enabled a paradigm shift from viewing CRS pathophysiology through the lens of airflow obstruction to a model in which the airway epithelium plays a crucial role.24
The airway and nasal epithelia orchestrate the complex interactions between the body’s external and internal environments.25,26 The nasal passage is the first point of entry for environmental triggers (such as pathogens, allergens, and pollutants) and odorants entering the respiratory tract that interact with the highly specialized nasal and olfactory epithelia, respectively.26–28
The healthy nasal epithelium maintains homeostasis through a variety of immune and non-immune functions, including acting as a physical barrier to environmental agents, driving mucociliary clearance, orchestrating innate immune responses through pattern-recognition receptors, and regulating adaptive immune cells via the release of cytokines.26
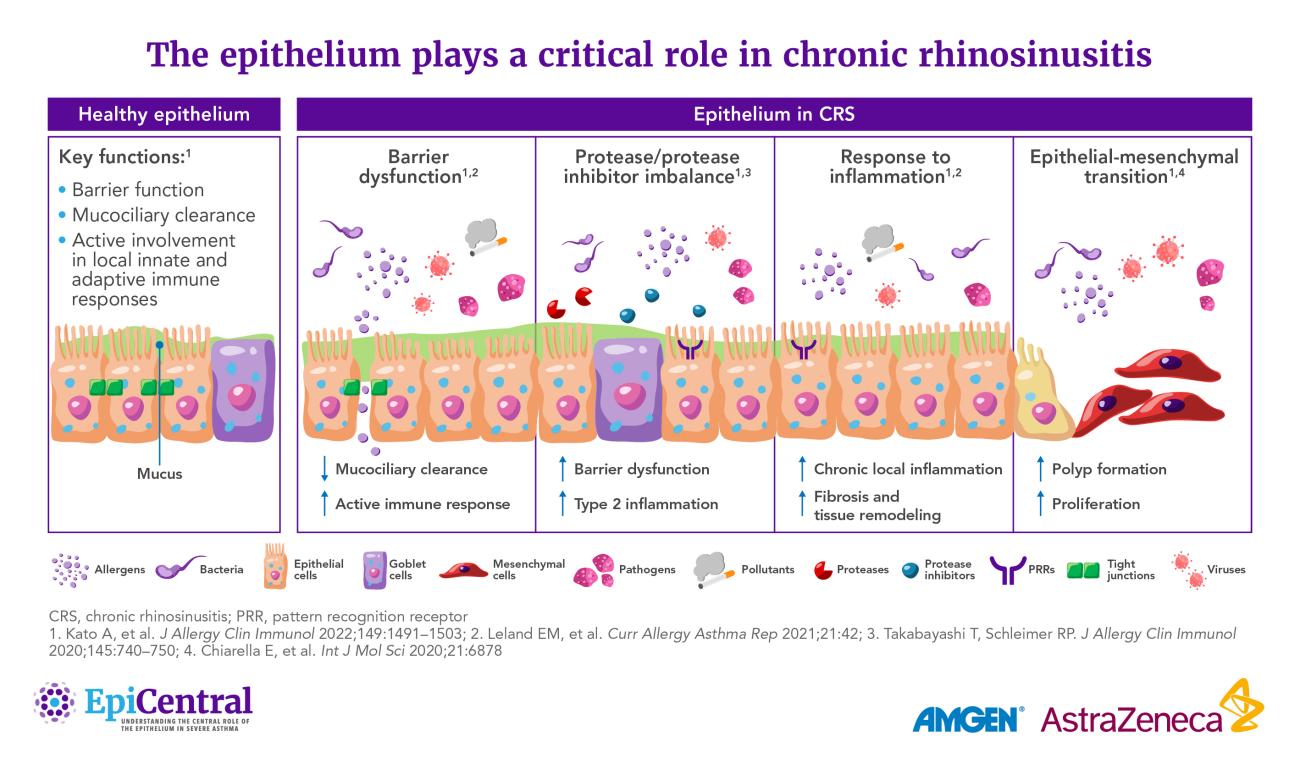
However, dysregulation of the epithelium is an important characteristic of CRS whereby the ability of the epithelium to act as a robust physical and immune barrier against the external environment becomes impeded:1
- There is excess secretion and altered composition of mucus, mainly associated with glandular hyperplasia in CRSsNP and goblet cell hyperplasia in CRSwNP29
- Reduced ciliary beating, in conjunction with excess mucus, impairs mucociliary clearance, contributing to chronic infection and inflammation26,30
- The olfactory epithelium also appears to be altered in CRS.31 Olfactory dysfunction is a key predictor of reduced quality of life in patients with CRSwNP.32 In part, anosmia/hyposmia may be caused by reduced airflow due to physical obstruction by nasal polyps.31 However, there is also evidence that inflammation within the olfactory epithelium leads to the impairment or death of sensory neurons, and hence the loss of sense of smell31
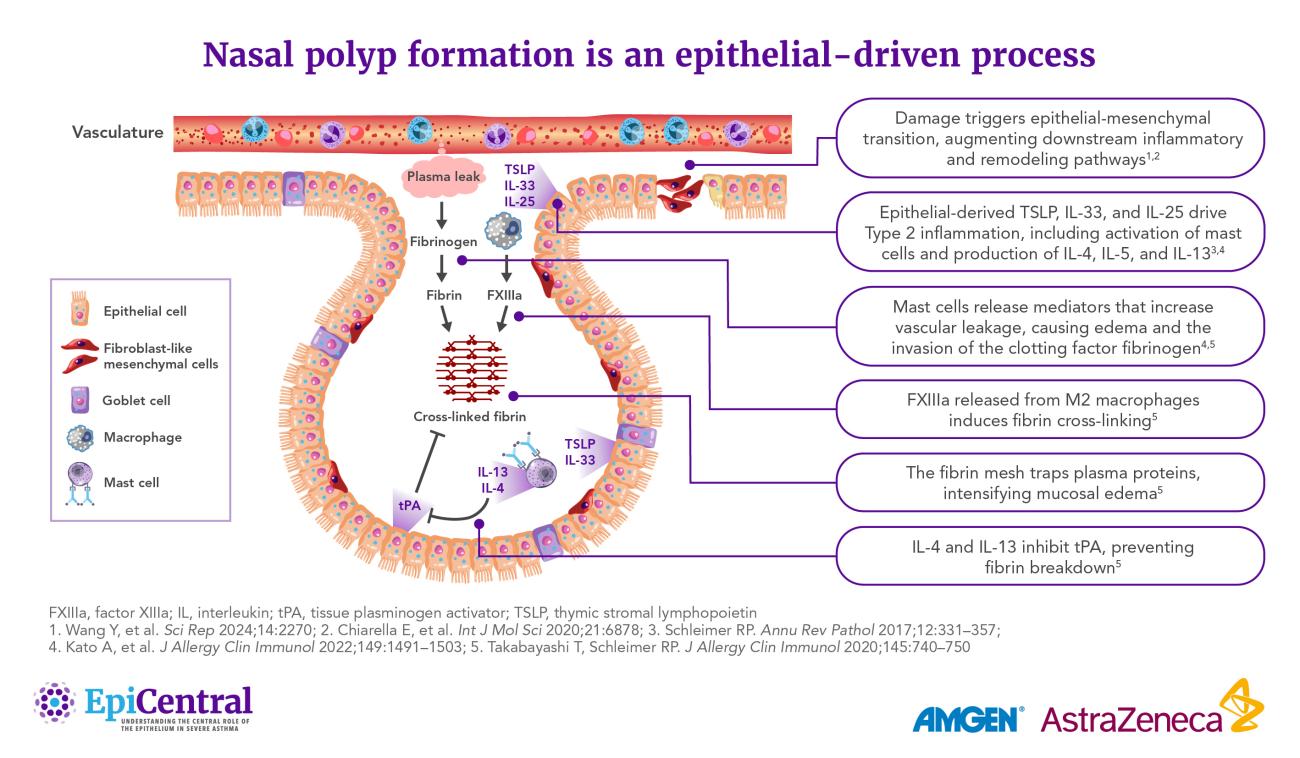
- Recent evidence indicates that damage to the epithelium may initiate nasal polyp formation through the process of epithelial-mesenchymal transition (EMT).33 In response to damage, epithelial cells can proliferate and undergo EMT, transforming into fibroblasts that promote tissue remodeling and, ultimately, polyp formation33
Understanding the mechanisms of pathogenesis is critical to providing optimized patient care, but this is challenging because of the highly heterogeneous nature of CRS.26,36
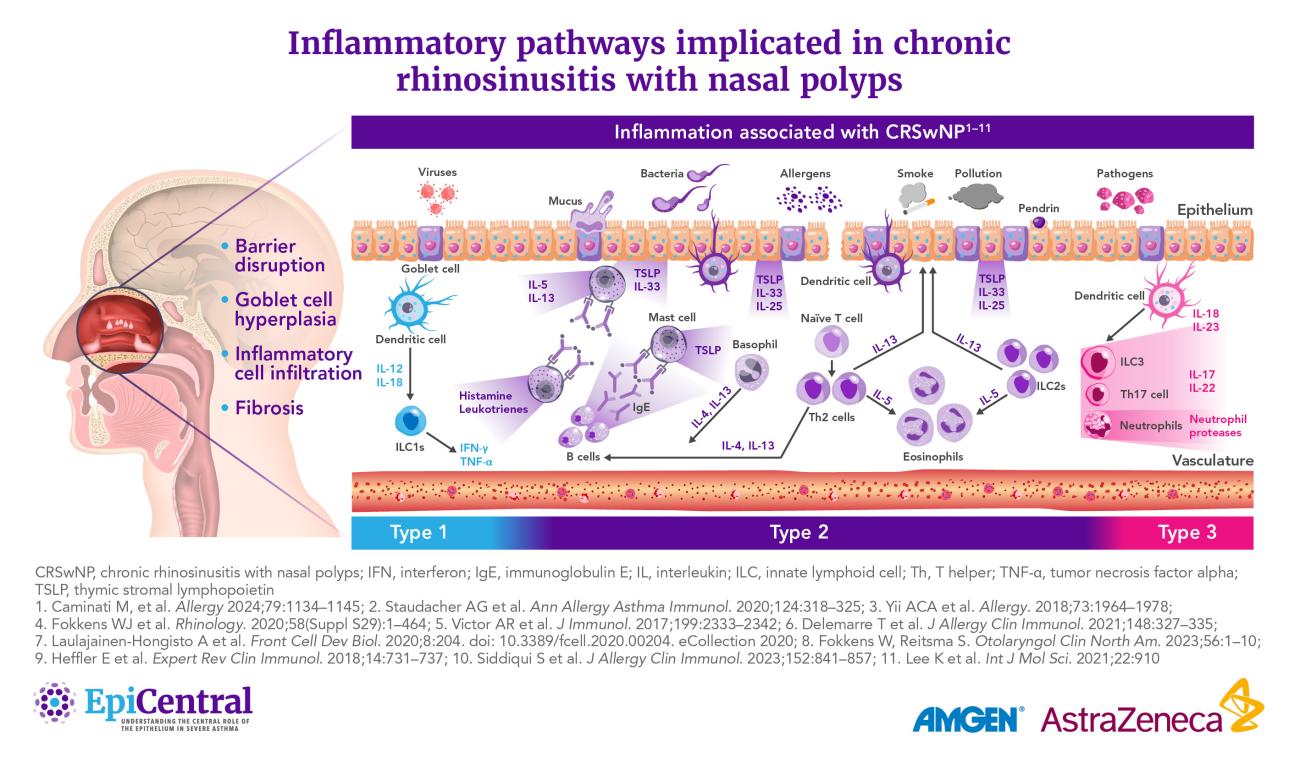
It was previously thought that CRSsNP and CRSwNP were characterized by distinct inflammatory endotypes, the former by Type (T)1 inflammation and the latter by T2 inflammation.26 However, recent studies show that both phenotypes can manifest in three main endotypes: T1, T2, or T3 inflammation.26,36 These endotypes are characterized by their profile of elevated cytokines and immune cells.36
Adding additional layers of complexity, a number of patients present with a mixed endotype or no clear endotype,37 and endotype prevalence appears to vary geographically:
- T2 is the predominant endotype of CRSwNP in Western countries, including the USA37
- Asian countries show higher proportions of T1 and T3 endotypes of CRSwNP.38,39 However, there has been an increasing prevalence of T2 inflammation in the past two decades, possibly owing to shifting environmental factors38,39
The epithelium can regulate various mediators associated with the endotypes of CRS, such as T2 cytokines, through the release of the epithelial cytokines thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33.40,41 These cytokines play key roles in the initiation of inflammatory pathways and structural changes underlying respiratory diseases such as asthma.42,43
Elevated expression of TSLP, IL-33, and IL-25 has also been demonstrated in the nasal epithelium of patients with CRSwNP.6 Levels of TSLP, the TSLP receptor (TSLPR), and the IL-33 receptor (ST2L) have been shown to correlate not only with markers of T2 inflammation in CRSwNP, but also with markers of disease severity in eosinophilic CRSwNP.7
CRS and asthma are both associated with epithelial barrier dysfunction and shared profiles of inflammatory cytokines.45
This shared pathogenesis is evidence toward the “united airways disease” concept, which describes how inflammatory diseases of the upper and lower airways frequently co-occur and share similar pathophysiology.45
In regard to frequent comorbidity, estimates suggest that 40–67% of patients with CRSwNP have comorbid asthma41 and asthma severity is positively correlated with CRS severity (as measured by Lund-Mackay scores).46 Similarly, in asthmatics, CRS is associated with increased asthma exacerbation frequency47 and is an independent negative predictor of quality of life.48
Learn more about the importance of the epithelium and epithelial cytokines in uniting upper and lower airway diseases.
A number of other comorbidities, such as allergy and COPD, are more frequent in patients with CRS than in controls.49
Learn more about the role of the epithelium and epithelial cytokines in COPD.
RELATED RESOURCES
Like this page? We have a host of other resources available in the ‘Scientific and Resource Library’ where you can find out more.
References
1. Wynne M, et al. Am J Rhinol Allergy 2019;33:782–790. 2. Orlandi RR, et al. Int Forum Allergy Rhinol 2021;11:213–739. 3. Sedaghat AR, et al. J Allergy Clin Immunol Pract 2022;10:1395–1403. 4. Benjamin MR, et al. J Allergy Clin Immunol Pract 2019;7:1010–1016. 5. Claeys N, et al. Front Allergy 2021;2:761388. 6. Zhang M, et al. Int Immunopharmacol 2023;121:110559. 7. Liao B, et al. Allergy 2015;70:1169–1180. 8. Cho SH, et al. J Allergy Clin Immunol Pract 2020;8:1505–1511. 9. Fokkens WJ, et al. Rhinology 2020;58(Suppl S29):1–464. 10. Soler ZM, et al. Laryngoscope 2011;121:2672–2678. 11. Schneider S, et al. J Clin Med 2020;9:925. 12. Kim J-Y, et al. JAMA Otolaryngol Head Neck Surg 2019;145:313–319. 13. Tomoum MO, et al. Int Forum Allergy Rhinol 2015;5:674–681. 14. Hopkins C, et al. Rhinology 2015;53:18–24. 15. Hopkins C, et al. Rhinology 2015;53:10–17. 16. Vennik J, et al. BMJ Open 2019;9:e022644. 17. Bhattacharyya N, et al. Laryngoscope 2019;129:1969–1975. 18. Hellings PW, et al. Rhinology 2023;61:85–89. 19. De Corso E, et al. J Pers Med 2022;12:897. 20. Price DB, et al. J Asthma Allergy 2018;11:193–204. 21. Fokkens WJ, et al. Allergy 2019;74:2312–2319. 22. van der Veen J, et al. Allergy 2017;72:282–290. 23. DeConde AS, et al. Laryngoscope 2017;127:550–555. 24. Yan B, et al. J Allergy Clin Immunol 2024;153:1206–1214. 25. Vroling AB, et al. Allergy 2008;63:1110–1123. 26. Kato A, et al. J Allergy Clin Immunol 2022;149:1491–1503. 27. Ha J-G, Cho H-J. Int J Mol Sci 2023;24:14229. 28. Harkema JR, et al. Toxicol Pathol 2006;34:252–269. 29. Tu Y, et al. Inflammation 2021;44:1937–1948. 30. Gudis D, et al. Am J Rhinol Allergy 2012;26:1–6. 31. Yan X, et al. Laryngoscope Investig Otolaryngol 2020;5:992–1002. 32. Mullol J, et al. J Allergy Clin Immunol Pract 2022;10:1434–1453.e9. 33. Wang Y, et al. Sci Rep 2024;14:2270. 34. Gong X, et al. Front Immunol 2023;14:1238673. 35. Chan Y, et al. J Otolaryngol Head Neck Surg 2023;52:50. 36. Staudacher AG, et al. Ann Allergy Asthma Immunol 2020;124:318–325. 37. Stevens WW, et al. J Allergy Clin Immunol Pract 2019;7:2812–2820.e3. 38. Zhang Y, et al. J Allergy Clin Immunol 2017;140:1230–1239. 39. Ryu G, et al. Precis Future Med 2022;6:170–176. 40. Schleimer RP. Annu Rev Pathol 2017;12:331–357. 41. Laidlaw TM, et al. J Allergy Clin Immunol Pract 2021;9:1133–1141. 42. Gauvreau GM, et al. Allergy 2023;78:402–417. 43. Duchesne M, et al. Front Immunol 2022;13:975914. 44. Cho SH, et al. J Allergy Clin Immunol Pract 2016;4:575–582. 45. Fokkens W, Reitsma S. Otolaryngol Clin North Am 2023;56:1–10. 46. Lin DC, et al. Am J Rhinol Allergy 2011;25:205–208. 47. Denlinger LC, et al. Am J Respir Crit Care Med 2017;195:302–313. 48. Ek A, et al. Allergy 2013;68:1314–1321. 49. Khan A, et al. Rhinology 2019;57:32–42.